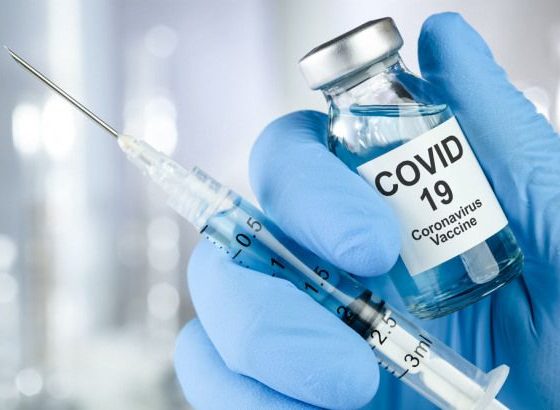
With the race towards finding a vaccine against Covid-19 intensifying by the day. There remains a question as to when the world will get the much-needed antidote to the deadly contagion. The COVID 19 vaccine trials in India are progressing well. India is eyeing the Oxford-AstraZeneca vaccine candidate as the likely first shot against Covid-19.
Dr. V.K. Paul
The Indian government said. Dr. V.K. Paul, a member of NITI Aayog and head of the government’s expert group on the COVID vaccine. They are progressing well and their development is going in a reassuring way.
However, the Oxford candidate – for whom the Pune-based serum institute is a manufacturing partner of British pharma. “If the vaccine gets the nod, it makes sense to use it,” said a source.
While the Serum begins the advanced phase of human trials (Phase 2 and 3) in India. Over the age of 18 in 17 selected sites across the country, India Biotech’s Kovaxin, jointly Developed with ICMR. And Zykov D of Zydus Cadila is in early stages 1 and 2.
The Serum’s trial is so far the largest for the Covid-19 vaccine in the country. The other companies have around 1,000-1,100 participants enrolled in five to eight sites. Top US expert Anthony Fauci said that he believes that the vaccine should be out by the end of this year. Fauci said that by the end of 2021 even half an effective vaccine would be sufficient to control the epidemic.
The Oxford candidate has already completed initial phases (Phase 1 and 2) of human trials in the UK and has shown positive results. For example, preliminary results have shown that the vaccine induces an antibody response in a similar range in individuals recovering from Covid-19 within 28 days. A second “booster” dose of the vaccine increased the antibody’s response to higher levels, and 100% of the blood sample showed neutralizing activity to the second dose.
The Oxford candidate scored better than other vaccines developed outside India as it has an Indian company, Serum Institute, as a manufacturing and distribution partner. Besides, Bill and Melinda Gates Foundation along with GAVI has also announced funding for the creation of early shots of this vaccine by Serum for India.
Dr. Paul said that the government consulted with the vaccine manufacturers so that they could learn about the facilities expected from the government.
“We requested vaccine manufacturers to indicate what the possible prices could be. Pricing is very complex because some of these vaccines are in infancy. We have some insight into what the price range may be. But it is information that refined as we go along, ”he said.
He further noted that individual vaccine manufacturers requested to provide more clear data on their individual production capabilities. And how their capacity will dissipate over time.
“This is a dialogue in motion,” he added.